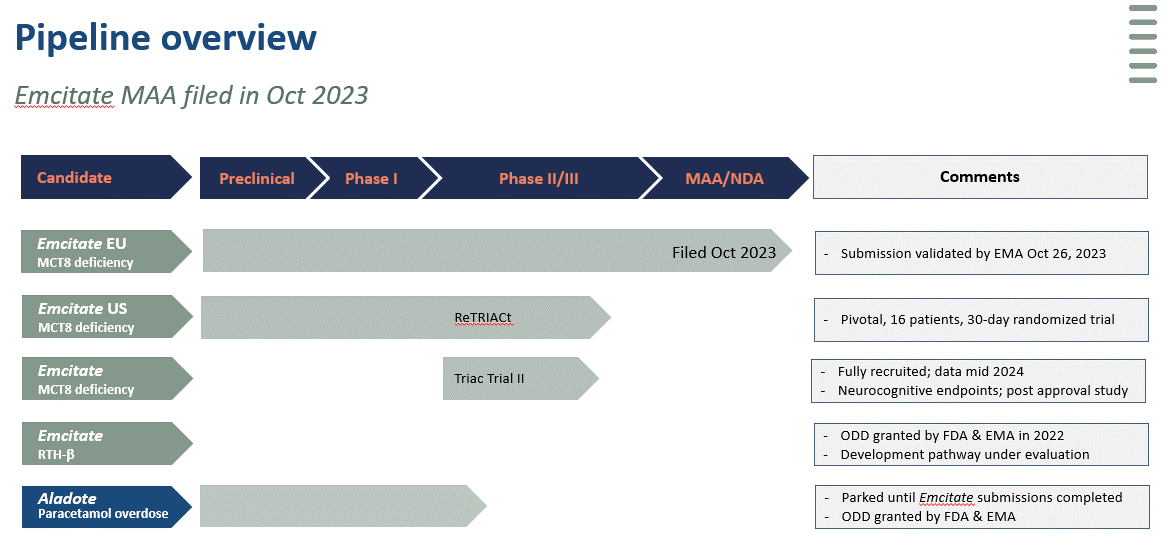
Pipeline
Emcitate®
Emcitate is under development for the treatment of patients with MCT8 deficiency, a highly debilitating rare disease with no available treatment. In previous studies (Triac Trial I and a long-term real-life study) Emcitate has shown highly significant and clinically relevant results on serum thyroid hormone T3 levels and secondary clinical endpoints. As a result of regulatory interaction Egetis submitted a marketing authorisation application (MAA) for Emcitate to the European Medicines Agency (EMA) in October 2023.
In the US, after discussions with the FDA, Egetis is conducting a small randomized, placebo-controlled study in 16 evaluable patients to verify the results on T3 levels seen in previous clinical trials and publications. Egetis intends to submit a new drug application (NDA) in the US for Emcitate in mid 2024 under the Fast-Track Designation granted by FDA.
Emcitate holds Orphan Drug Designation (ODD) for MCT8 deficiency and resistance to thyroid hormone type beta (RTH- β) in the US and the EU. Emcitate has been granted Rare Pediatric Disease Designation (RPD) which gives Egetis the opportunity to receive a Priority Review Voucher (PRV) in the US, after approval.
Aladote®
Aladote® a first-in-class drug candidate, is being developed to reduce the risk of acute liver injury associated with acetaminophen/paracetamol poisoning. A proof of principle study has been completed. Design of pivotal Phase IIb/III study for Aladote® finalized after completed interactions with FDA, EMA and MHRA. Aladote® has been granted Orphan Drug Designation in the US and EU.